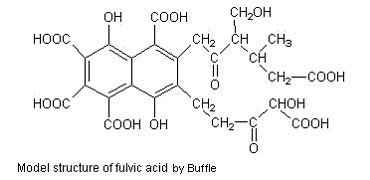
Humic acid and fulvic acid can interact with heavy metals. The results show that under acidic conditions, when there is hexavalent chromium ion in solution, fulvic acid will reduce Cr (VI) to Cr
(III). And then chelate with Cr (III). The chelate has certain binding ability to sand particle surface. Which shows the adsorption ability of chromium ion.
At the same time, fulvic acid is bound to the surface active position of sand particles to form a stronger ion exchange center for trace metals. Which strengthens the adsorption of heavy metals by sand particles.The results of researching show that humic acids also has the effect of reduction and chelation. It also has a certain adsorption capacity for heavy metals.
Therefore, under acidic conditions, it affects the adsorption properties of heavy metals on sand particles by humic acid and fulvic acid.
Some experiments also show that the removal rate of water-soluble chromium can reach 50% in the presence of humic acid and 22% in the absence of humic acid. It can be seen that humic acids can obviously enhance the adsorption capacity of heavy metal ions in sand.
Jiao Wentao and other studies show that with the decrease of humic acid content in soil. The adsorption capacity of chromium decreases correspondingly. But the desorption rate increases in varying degrees. Which indicates that humic acids changes the surface electric charge of soil. Promotes the chemical binding and fixation of heavy metals in soil.