Humus is a complex organic matter formed by animals and plants through long-term physical, chemical and biological actions. Water body sediment and soil contain humus. Humic acid powder and granular are macromolecule polymers with complex chemical structure. They all have carboxyl, phenol, ketone groups and other active groups.
Their molecular weights range from 102 to 106. Humus can be divided into humic acid (HA), fulvic acid (FA) and humin(HM) according to its solubility in acid and alkali. HA is the main organic substance in natural drinking water. Its concentration ranges from 20 ug/L of groundwater to 30 mg/L of surface water. The higher the content, the worse the sanitary condition of water quality.

The content of humic acid in general water sources is about 10 mg/L, accounting for 50%~90% of total organic matter in water. The existence of humic acid in natural drinking water sources has brought a series of effects on human beings, animals and plants.
Effect of humic acid on drinking water
(1) HA is a complexing agent of trace metal elements. The existence of humic acid, on the one hand, will reduce the content of metal ions and trace elements in water and the mineralization, thus destroying the adsorption and balance of some elements such as Ca, Mg, Mn, V, Mo, SO2-4, etc. on the other hand, it can affect the toxicity and biological effectiveness of metal ions.
(2) Humic acids in water are important precursors of halogenation by-products. Humus is easy to form disinfection by-products DBPs and THMs during chlorination in water plants. It has been reported that almost all natural aquatic organisms may be chlorinated during disinfection.
Humic acid, which accounts for about half of dissolved aquatic organisms, is the most important precursor for THMs production. Studies have shown that dissolved humic acids are the main precursors of MX formation in natural waters. Some phenols, aldehydes and aromatic acids may play an important role in the formation of MX.
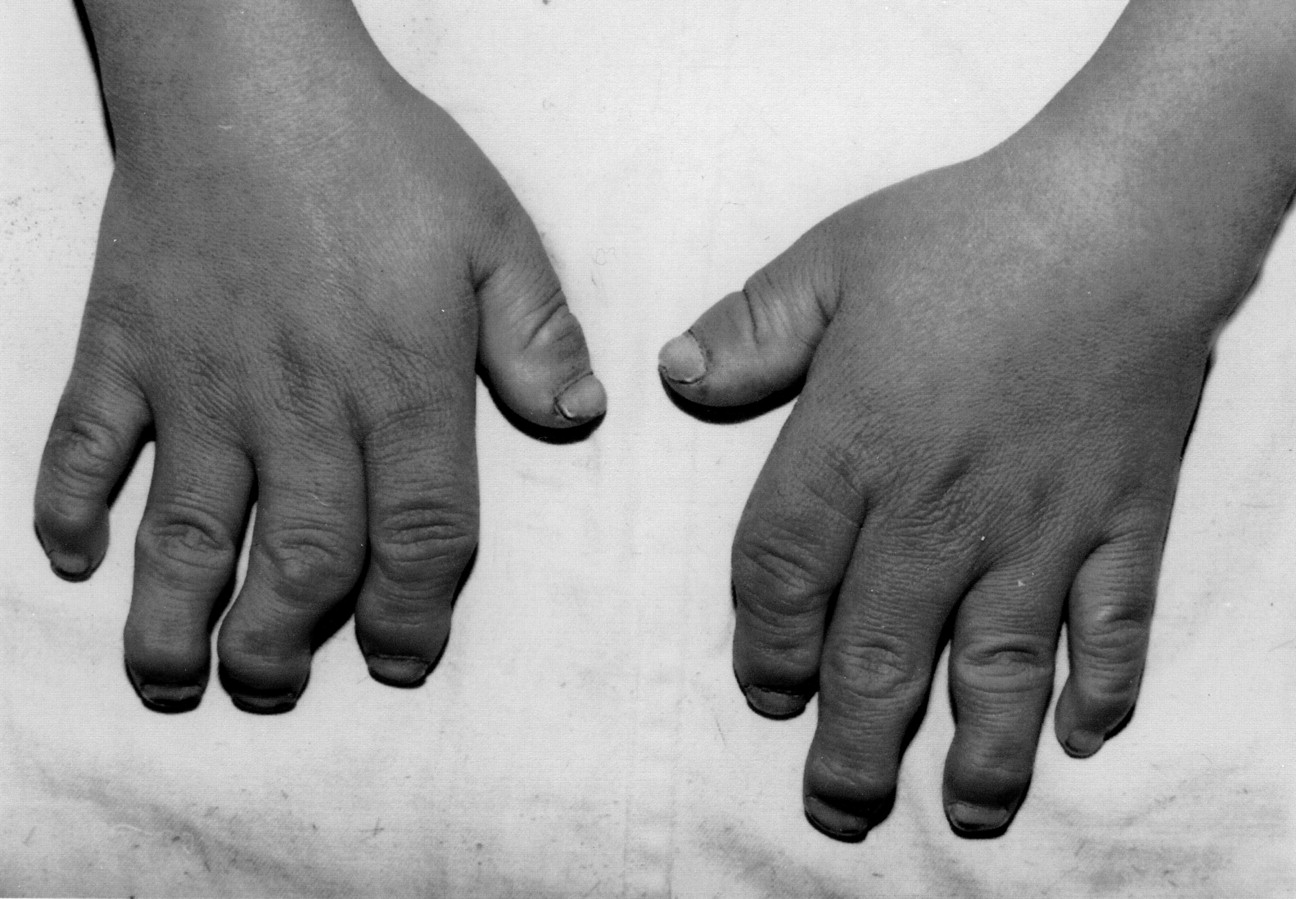
(3) Humic acid is one of the main environmental factors leading to Kashin-Beck disease. Drinking water of residents in Kashin-Beck disease area is often dark and humid pit water, which can not get enough sunlight, and the content of humic acid in water is high because of less photolysis.
(4) Water acidification causes changes in the characteristics of humus (HS), thus affecting the environment. With the deterioration of human living environment, the formation of acid rain, lakes and other natural water body pH decline.
As the organic nitrogen content of HS in water increases, the proportion of hydrophobic substances to hydrophilic substances decreases, the acidity of carbon and carboxyl groups decreases, the acidity of oxygen and phenol and aldehyde increases, which leads to the increase of toxicity of fish and the primary production of phytoplankton, the disappearance of some major zooplankton species, the decrease of macrophytes and the increase of epiphytes in the treated watershed.